Theoretical investigation on the chemoselective N-heterocyclic carbene-catalyzed cross-benzoin reactions†
Abstract
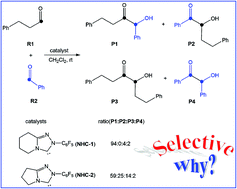
A density functional theory study was performed to understand the detailed mechanisms of the cross-benzoin reactions catalyzed by N-heterocyclic carbene (NHC) species. Our theoretical study predicted that the first H-transfer operates with water in solution as a mediator, and the second H-transfer undergoes a concerted mechanism rather than a stepwise one. In addition, the chemoselectivity of the reactions studied in this work has been explored. P1 was obtained as a major product mainly due to the more stable intermediate formed by reaction of NHC with reactant R1. Different steric effects resulting from the fused six-membered ring in transition state TS7 and the fused five-membered ring in transition state TS13 are the origin leading to the chemoselectivity.

 Please wait while we load your content...
Please wait while we load your content...