Improving catalyst activity in secondary amine catalysed transformations†
Abstract
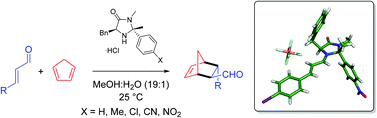
The effect on catalyst performance of altering substituents at the 2-position of the Macmillan imidazolidinone has been examined. Condensation of L-phenylalanine N-methyl amide with acetophenone derivatives results in a series of imidazolidinones whose salts can be used to accelerate the Diels–Alder cycloaddition. Electron withdrawing groups significantly increase the overall rate of cycloaddition without compromise in selectivity. The most effective catalyst was shown to be efficient for a variety of substrates and the applicability of this catalyst to alternative secondary amine catalysed transformations is also discussed.

 Please wait while we load your content...
Please wait while we load your content...