Synthesis of PS/PO-chimeric oligonucleotides using mixed oxathiaphospholane and phosphoramidite chemistry†
Abstract
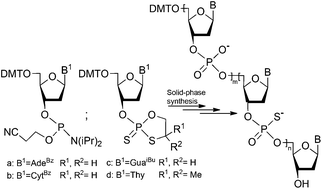
Chimeric oligonucleotides containing phosphodiester and phosphorothioate linkages have been obtained using the solid phase synthesis. The oligonucleotide parts possessing natural internucleotide phosphate bonds were assembled using commercially available nucleoside 3′-O-(2-cyanoethyl-N,N-diisopropylamino)phosphoramidites 7 whereas the phosphorothioate segment was built using nucleoside 3′-O-(2-thio-1,3,2-oxathiaphospholanes) 3. The oxidation steps, crucial for the conversion of phosphite linkages into the phosphate moieties, were conducted using tert-butylperoxy-trimethylsilane, and this reagent was not harmful to the diester phosphorothioate linkages. When P-diastereopure nucleoside 3′-O-(2-thio-1,3,2-oxathiaphospholane) monomers were employed the resulting chimeric backbone retained the P-stereoregularity of the phosphorothioate units.

 Please wait while we load your content...
Please wait while we load your content...