Pd-catalyzed oxidative C–H alkenylation for synthesizing arylvinyltriazole nucleosides†
Abstract
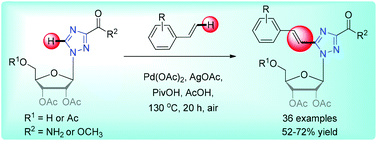
Various arylvinyltriazole nucleoside analogues were synthesized using Pd-catalyzed oxidative Heck reaction. This method affords the corresponding and otherwise difficult to achieve arylvinyltriazole nucleosides with good yields and large functional group compatibility. These results further advocate the potential and practicality of this oxidative C–H alkenylation method for generating structurally challenging chemical entities in organic synthesis.

 Please wait while we load your content...
Please wait while we load your content...