PEGylation enhances the tumor selectivity of melanoma-targeted conjugates†
Abstract
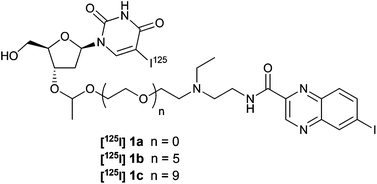
In the development of our melanoma-selective delivery approach, three preselected conjugates of 5-iodo-2′-deoxyuridine (IUdR) to the ICF01012 melanoma-carrier were radiolabelled with iodine-125, and their in vivo distribution profile was determined. A radioiodination method for the conjugate 1a and its PEGylated derivatives 1b–c was developed via electrophilic iododestannylation in good radiochemical yield with excellent radiochemical purity (>99%). When administered to melanoma-bearing mice, the PEGylated conjugates exhibited an increased tumour uptake with a prolonged residence time. PEGylation also resulted in enhanced tumour selectivity compared with the non-PEGylated parent. These characteristics support further development of this model to achieve maximal concentration of anticancer therapeutics at the local site of action and minimize distribution to non-targeted sites.

 Please wait while we load your content...
Please wait while we load your content...