Synthesis of coenzyme A thioesters using methyl acyl phosphates in an aqueous medium†
Abstract
Regioselective S-acylation of coenzyme A (CoA) is achieved under aqueous conditions using various aliphatic and aromatic carboxylic acids activated as their methyl acyl phosphate monoesters. Unlike many hydrophobic activating groups, the anionic methyl acyl phosphate mixed anhydride is more compatible with aqueous solvents, making it useful for conducting acylation reactions in an aqueous medium.
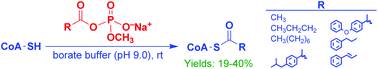

 Please wait while we load your content...
Please wait while we load your content...