Carbohydrate-based N-heterocyclic carbenes for enantioselective catalysis†
Abstract
Versatile syntheses of C2-linked and C2-symmetric carbohydrate-based imidazol(in)ium salts from functionalised amino-carbohydrate derivatives are reported. The novel NHCs were ligated to [Rh(COD)Cl]2 and evaluated in Rh-catalysed asymmetric hydrosilylation of ketones with good yields and promising enantioselectivities.
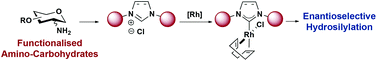

 Please wait while we load your content...
Please wait while we load your content...