Intramolecular redox reaction for the synthesis of N-aryl pyrroles catalyzed by Lewis acids†
Abstract
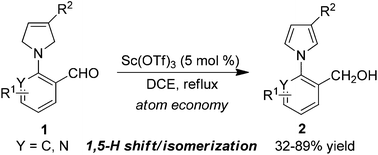
An efficient approach to synthesize N-aryl pyrroles via Lewis acid-mediated 1,5-hydride shift and isomerization of 2-(3-pyrroline-1-yl)arylaldehydes has been achieved in up to 89% yield. This methodology is applicable to the synthesis of fluorazene derivatives as electron donor (D)/acceptor (A) molecules.

 Please wait while we load your content...
Please wait while we load your content...