Regioselective ruthenium catalysed H–D exchange using D2O as the deuterium source†
Abstract
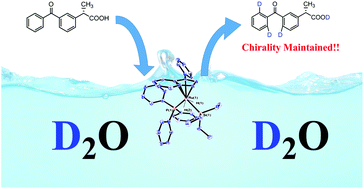
An efficient and convenient ruthenium catalysed method for a regiospecific H/D exchange using D2O is described. Organic moieties such as pyridine, oxazole, imidazole, pyrazole, ester, ketone and carboxylic acid have been found effective directing groups in this transformation. In addition, the deuteration of the enantiopure (S)-Ketoprofen leads to the incorporation of three deuterium atoms with retention of molecular chirality.
 Please wait while we load your content...
Please wait while we load your content...