Trienamines derived from 5-substituted furfurals: remote ε-functionalization of 2,4-dienals†
Abstract
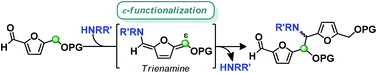
The selective ε-functionalization of 5-substituted furfurals via trienamine intermediates is reported herein. This methodology was successfully applied to several 5-substituted furfurals with different amines via formation of a trienamine through the furan ring. The rationalized reaction mechanism involves the addition of the trienamine intermediate to its corresponding iminium-ion producing new furan-containing scaffolds.

 Please wait while we load your content...
Please wait while we load your content...