Rhodium(ii) catalyzed synthesis of macrocycles incorporating oxindole via O–H/N–H insertion reactions†
Abstract
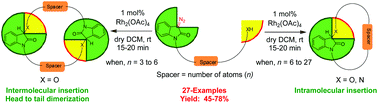
A wide variety of 10- to 29-membered oxaza-macrocycles incorporating an oxindole unit were synthesized in good yield via rhodium(II) acetate dimer catalyzed intramolecular O–H/N–H insertion reactions. Interestingly, synthesis of C2-symmetric macrocycles in moderate yield was also demonstrated via head to tail dimerization involving double intermolecular O–H insertion when the spacer length was decreased. The synthesis of chiral macrocycles was also delineated. This study reveals the effect of spacer length on inter- or intramolecular insertion reactions with the remotely placed hydroxyl/amino group.

 Please wait while we load your content...
Please wait while we load your content...