Oxidative nucleophilic strategy for synthesis of thiocyanates and trifluoromethyl sulfides from thiols†
Abstract
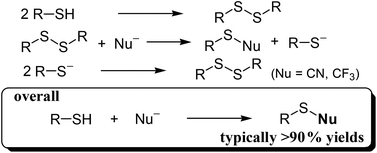
Thiocyanates and trifluoromethyl sulfides are important compounds and have classically been synthesized via multistep procedures together with the formation of significant amounts of byproducts. Herein, we demonstrate an oxidative nucleophilic strategy for the synthesis of thiocyanates and trifluoromethyl sulfides from thiol starting materials using nucleophilic reagents such as TMSCN and TMSCF3 (TMS = trimethylsilyl). In the presence of a 2 × 2 manganese oxide-based octahedral molecular sieve (OMS-2) and potassium fluoride (KF), various structurally diverse thiocyanates and trifluoromethyl sulfides could be synthesized in almost quantitative yields (typically >90%). The presented cyanation and trifluoromethylation reactions proceed through the OMS-2-catalyzed oxidative homocoupling of thiols to give disulfides followed by nucleophilic bond cleavage to produce the desired compounds and thiolate species (herein S-trimethylsilylated thiols). OMS-2 can catalyze oxidative homocoupling of the thiolate species, thus resulting formally in the quantitative production of thiocyanates and trifluoromethyl sulfides from thiols.

 Please wait while we load your content...
Please wait while we load your content...