Virtual screening and biological evaluation of novel small molecular inhibitors against protein arginine methyltransferase 1 (PRMT1)†
Abstract
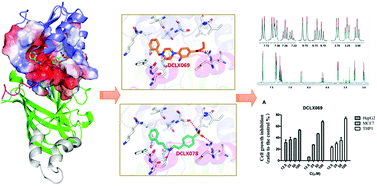
Protein arginine methylation is a common post-translational modification which is crucial for a variety of biological processes. Dysregulation of protein arginine methyltransferases (PRMTs) activity has been implicated in cancer and other serious diseases. Thus, small molecule inhibitors against PRMT have great potential for therapeutic development. Herein, through the combination of virtual screening and bioassays, six small molecular compounds were identified as PRMT1 inhibitors. Amongst them, the binding affinity of compounds DCLX069 and DCLX078 with PRMT1 was further validated by T1ρ and saturation transfer difference (STD) NMR experiments. Most important of all, both compounds effectively blocked cell proliferation in breast cancer, liver cancer and acute myeloid leukemia cell lines. The binding mode analysis from molecular docking simulations theoretically indicated that both inhibitors occupied the SAM binding pocket to exert the inhibitory effect. Taken together, our compounds enriched the structural scaffolds as PRMT1 inhibitors and afforded clues for further optimization.

 Please wait while we load your content...
Please wait while we load your content...