First total synthesis of antihypertensive natural products S-(+)-XJP and R-(−)-XJP†
Abstract
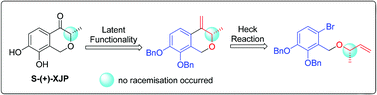
The first asymmetric total synthesis of antihypertensive natural products S-(+)-XJP and R-(−)-XJP has been achieved in 8 steps starting from commercially available 6-bromo-2-hydroxy-3-methoxybenzaldehyde. Key steps included intramolecular Heck reaction and oxidative ozonolysis reaction with the retention of stereochemistry. A latent functionality strategy was implemented to circumvent the racemization in this endeavor. The protocol described here provided a fast and easily accessible synthetic method to obtain optically pure isochroman-4-one derivatives. Furthermore, the in vivo antihypertensive effects of (±)-XJP, S-(+)-XJP and R-(−)-XJP were investigated on spontaneously hypertensive rats. The obtained results could provide valuable information to identify a promising lead for further chemical modification research.

 Please wait while we load your content...
Please wait while we load your content...