Preparation of indium nitronates and their Henry reactions†
Abstract
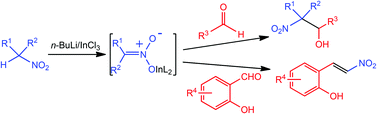
Indium nitronates were readily prepared from commercially available nitroalkanes by transmetallation of the corresponding lithium nitronates with indium salts. The Henry reaction of this indium organometallics with aldehydes afforded β-nitroalkanols in moderate to high yields. The use of chiral sugar aldehydes furnished the corresponding carbohydrate-derived β-nitroalkanols with excellent stereoselectivity.
 Please wait while we load your content...
Please wait while we load your content...