Asymmetric total synthesis of paecilomycin E, 10′-epi-paecilomycin E and 6′-epi-cochliomycin C†
Abstract
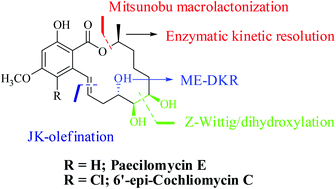
The asymmetric total syntheses of naturally occurring resorcylic acid lactone paecilomycin E and two of its structural congeners have been reported in this article. The major highlight of the synthetic venture is the application of the late stage Mitsunobu macrolactonization method (as it is difficult to achieve the desired products through the standard carboxyl activation method) of a properly functionalized seco-acid. The macrolactonization precursor was synthesized by applying an “E”-selective Julia–Kocienski olefination of a highly functionalized aromatic aldehyde and a sulphone, which constitutes all the stereocenters (C4′, C5′, C6′ and C10′; 3S,7R,8R,9S) in the target molecule.

 Please wait while we load your content...
Please wait while we load your content...