Highly diastereoselective 1,3-dipolar cycloadditions of chiral non-racemic nitrones to 1,2-diaza-1,3-dienes: an experimental and computational investigation†
Abstract
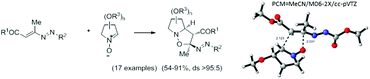
Asymmetric 1,3-dipolar cycloadditions between 1,2-diaza-1,3-dienes and chiral non-racemic nitrones to give 3-substituted-5-diazenyl isoxazolidines were studied both experimentally and theoretically. Whereas cyclic nitrones provide complete selectivity for the cycloaddition reaction (only one isomer is obtained), acyclic nitrones derived from D-glyceraldehyde and D-galactose lead to 1 : 1 mixtures of two isomers. A DFT analysis based on reactivity indices correctly predicts the regiochemistry of the reaction in agreement with the high electron-withdrawing character of the diazenyl group. The same theoretical studies considering solvent effects (PCM model) based on transition state theory are in qualitative agreement with the observed experimental results.

 Please wait while we load your content...
Please wait while we load your content...