Synthesis and SARs of indole-based α-amino acids as potent HIV-1 non-nucleoside reverse transcriptase inhibitors†
Abstract
A series of non-nucleoside reverse transcriptase inhibitors derived from indole-based α-amino acids were designed and synthesized. Their inhibitory activities were detected by a TZM-bl cell assay on HIV virus type HIV-1IIIB. The comprehensive understanding of the SAR was obtained by utilizing the variation of the substituents of the indole-based α-amino acids. From the screened compounds, the novel inhibitors 19 and 29 were identified to be highly potent candidates with EC50 values of 0.060 μM and 0.045 μM respectively (CC50 values of 109.545 μM and 49.295 μM and SI values of 1825.8 and 1095.4). In most cases, the variation of substituents at different positions had a significant effect on the potency of activities. The results also indicate that the indole-based α-amino acids as efficient NNRTIs displayed comparable anti-HIV-1 activities to the reference drug NVP. We hope the identification of these indole-based amino acids as efficient NNRTIs of RT could stimulate researchers to develop more diversified anti-HIV drugs.
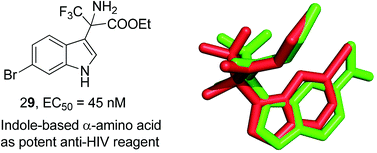

 Please wait while we load your content...
Please wait while we load your content...