Ultrasound-assisted 1,3-dipolar cycloaddition and cyclopropanation reactions for the synthesis of bis-indolizine and bis-cyclopropane derivatives†
Abstract
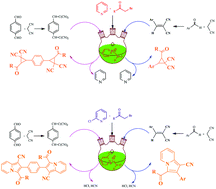
Ultrasound irradiation can promote the 1,3-dipolar cycloaddition reaction of 2-chloropyridinium ylides with 2-benzylidenemalononitrile or 2,2′-(1,4-phenylenebis(methanylylidene))dimalononitrile, to afford the indolizine and bis-indolizine derivatives respectively. While the reaction of pyridinium ylides with 2-benzylidenemalononitrile or 2,2′-(1,4-phenylenebis(methanylylidene))dimalononitrile under ultrasound irradiation provided, in an unusual manner, cyclopropane and bis-cyclopropane derivatives, respectively. These cycloaddition and cyclopropanation reactions were carried out in the presence of triethylamine, in acetonitrile, at room temperature.

 Please wait while we load your content...
Please wait while we load your content...