The first stereoselective total synthesis of neosemburin and isoneosemburin†
Abstract
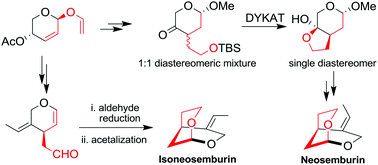
The Claisen rearrangement of sugar derived allyl vinyl ether, Wittig olefination and intramolecular acetalization reactions were used as key steps in the first stereoselective total synthesis of neosemburin and isoneosemburin through the formation of a 3-C-branched sugar precursor as a key intermediate.

 Please wait while we load your content...
Please wait while we load your content...