Pyridoxine-derived bicyclic amido-, ureido-, and carbamato-pyridinols: synthesis and antiangiogenic activities†
Abstract
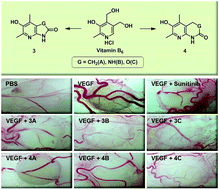
We recently developed an efficient and practical synthesis for a novel series of pyridoxine-derived 6-amido-2,4,5-trimethylpyridin-3-ols and found that this novel scaffold has outstanding activity to inhibit angiogenesis measured by the quantitative chick embryo chorioallantoic membrane (CAM) assay. As an effort to extend the scope of the amidopyridinol scaffold, we here report the synthesis and antiangiogenic activities of a series of bicyclic versions of the amidopyridinol including five- and six-membered cyclic amide-, cyclic urea-, and cyclic carbamate-fused pyridinols. The six membered bicyclic derivatives were prepared by the reported procedures, and the five-membered ring-fused ones were synthesized by new synthetic methods developed in this study. CAM assays showed that both six- and five-membered lactam-fused pyridinols have activities comparable to sunitinib malate, the positive control, in inhibition of vascular endothelial growth factor-induced angiogenesis. On the other hand, the urea and the carbamate derivatives showed modest to moderate antiangiogenic activities. In summary, some bicyclic aminopyridinols can provide a good platform for structural exploitation in future medicinal chemistry work.
 Please wait while we load your content...
Please wait while we load your content...