Palladium-catalyzed synthesis of isoindoloquinazolinones via dicarbonylation of 1,2-dibromoarenes†
Abstract
A convenient procedure for the carbonylative synthesis of isoindoloquinazolinones has been developed. Using 1,2-dibromobenzenes and 2-aminobenzyl amine as substrates and palladium as the catalyst, the desired products were isolated in moderate to good yields with the installation of two molecules of carbon monoxide. Notably, this is the first example of carbonylative synthesis of batracylin analogues.
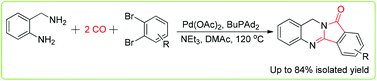

 Please wait while we load your content...
Please wait while we load your content...