Enantioselective palladium catalyzed conjugate additions of ortho-substituted arylboronic acids to β,β-disubstituted cyclic enones: total synthesis of herbertenediol, enokipodin A and enokipodin B†
Abstract
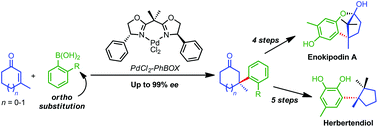
The palladium-catalyzed conjugate addition (Michael addition) of ortho-substituted arylboronic acids to β,β-disubstituted cyclic enones, in particular 3-methyl cyclopent-2-enone and 3-methyl cyclohex-2-enone, is reported. With an achiral bipyridine-based palladium catalyst, good yields are obtained with a variety of ortho-substituted arylboronic acids. In the asymmetric version, good to very high enantiomeric excesses (up to 99% ee) are obtained, though the yields are moderate. The decreased yields are attributed to significant protodeboronation of the arylboronic acid. The developed methodology allows the efficient enantioselective synthesis of the very crowded, biologically active, sesquiterpenes herbertenediol, enokipodin A, and enokipodin B.

 Please wait while we load your content...
Please wait while we load your content...