Highly enantioselective synthesis of bisoxindoles with two vicinal quaternary stereocenters via Lewis base mediated addition of oxindoles to isatin-derived ketimines†
Abstract
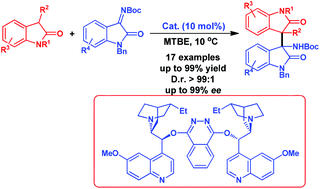
A highly efficient asymmetric organocatalytic addition of 3-substituted oxindole to isatin-derived ketimine is reported with excellent stereocontrol (>99 : 1 dr, >99% ee) under mild conditions. This method provides access to the bisoxindole structure moiety with two vicinal quaternary stereogenic centers.

 Please wait while we load your content...
Please wait while we load your content...