Synthesis and chemosensory application of water-soluble polyfluorenes containing carboxylated groups
Abstract
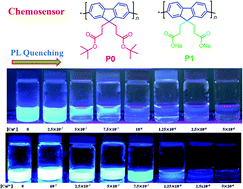
Detection of metal ions in aqueous solutions is a major issue for environmental protection. Conjugated polyelectrolytes showing high sensitivity and selectivity towards the detection of metal ions are highly desirable. We report a water-soluble polyfluorene containing carboxylated groups (P1), poly[9,9′-bis(3′′-propanoate)fluoren-2,7-yl] sodium salt, which shows high recognition capability toward Cu+ and Cu2+. P1 was prepared via the hydrolysis of poly[9,9′-bis(tert-butyl-3′′-propanoate)fluoren-2,7-yl] (P0) which was synthesized by Suzuki coupling polymerization. The photoluminescence (PL) spectra of P1 in aqueous solution are significantly quenched in the presence of Cu+ and Cu2+. P1 shows high selectivity and sensitivity toward Cu+ and Cu2+, with the Stern–Volmer constants (Ksv) being 3.5 × 106 and 5.78 × 106 M−1, respectively. Moreover, the stoichiometric ratio of the P1 repeat unit to Cu+ or Cu2+ is 2 : 1 obtained from Job's plot. P1 maintains high selectivity towards Cu+ or Cu2+ in the presence of various metal cations. Our results demonstrate that P1 shows very high sensitivity and selectivity in recognizing Cu+ and Cu2+, indicating that it is a promising functional material for chemical sensors.

 Please wait while we load your content...
Please wait while we load your content...