Brønsted acid mediated nitrogenation of propargylic alcohols: an efficient approach to alkenyl nitriles†
Abstract
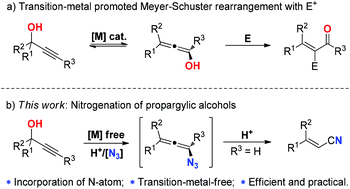
A novel and efficient approach to alkenyl nitriles from readily available propargylic alcohols has been developed. This nitrogenation reaction is transition-metal-free and could be conducted under air at ambient temperature, which makes this protocol promising and practical. Moreover, NH4Br is disclosed as an efficient additive to promote the stereoselectivity of this reaction.

 Please wait while we load your content...
Please wait while we load your content...