Diels–Alder reactions of 4-halo masked o-benzoquinones. Experimental and theoretical investigations†
Abstract
The studies on [4 + 2] cycloaddition of 4-halo derivatives of 6,6-dimethoxycyclohexa-2,4-dienones known as orthoquinone monoketals/masked o-benzoquinones are described. The 4-fluoro, 4-chloro- and 4-iodo-masked o-benzoquinones were stable enough for their isolation and characterization. These conjugated dienones cycloadded with several electron-deficient and electron-rich dienophiles in a highly regio- and stereo-selective manner to afford the corresponding halo bicyclo[2.2.2]octenone derivatives in high to excellent chemical yields. The halo masked o-benzoquinones did not undergo dimerization under the reaction conditions. To evaluate the observed selectivities of these Diels–Alder reactions, we have performed quantum mechanical calculations for the reactions between halo masked o-benzoquinones and methyl vinyl ketone and ethyl vinyl ether at the B3LYP/6-31G** theory level. The differences in HOMO and LUMO energy gaps suggest that these reactions can be classified as inverse electron-demand Diels–Alder reactions. The calculated transition state energies and global electronic indexes supported the experimentally observed selectivities of the reaction in many cases.
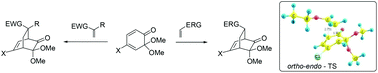

 Please wait while we load your content...
Please wait while we load your content...