Design, synthesis and biological evaluation of hydrogen sulfide releasing derivatives of 3-n-butylphthalide as potential antiplatelet and antithrombotic agents†
Abstract
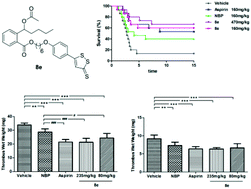
In the present study, a series of hydrogen sulfide (H2S) releasing derivatives (8a–g and 9a–f) of 3-n-butylphthalide (NBP) were designed, synthesized and biologically evaluated. The most promising compound 8e significantly inhibited the adenosine diphosphate (ADP) and arachidonic acid (AA)-induced platelet aggregation in vitro, superior to NBP, ticlopidine hydrochloride and aspirin. Furthermore, 8e could slowly produce moderate levels of H2S in vitro, which could be beneficial for improving cardiovascular and cerebral circulation. Most importantly, 8e protected against the collagen and adrenaline induced thrombosis in mice, and exhibited greater antithrombotic activity than NBP and aspirin in rats. Overall, 8e could warrant further investigation for the treatment of thrombosis-related ischemic stroke.

 Please wait while we load your content...
Please wait while we load your content...