A large dipole moment to promote gelation for 4-nitrophenylacrylonitrile derivatives with gelation-induced emission enhancement properties†
Abstract
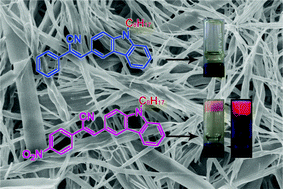
A series of 4-nitrophenylacrylonitrile and phenylacrylonitrile derivatives consisting of a carbazole moiety was synthesized. Some of these derivatives with longer alkyl chains and a nitro group could gelatinize some organic solvents, such as ethanol, n-butanol, ethyl acetate, and DMSO. By contrast, phenylacrylonitrile derivatives did not form gels in measured solvents. This result proved that the electron-withdrawing nitro moiety was important for gel formation because it conferred the molecules with large dipole moments, which enhanced the intermolecular interaction. Analyses by UV-vis absorption, X-ray diffraction, and scanning electron microscopy showed that the gelator molecules could self-assemble into one-dimensional nanofibers with layer packing, which further twisted into thicker fibers and formed three-dimensional networks in the gel phase. The single crystal structure of C4CNPA implied that the gelators might adopt an anti-parallel molecular stacking because of their larger ground-state dipole moment. Interestingly, the organogels had enhanced fluorescence relative to solutions at the same concentrations.

 Please wait while we load your content...
Please wait while we load your content...