Iron-catalyzed radical aryldifluoromethylation of activated alkenes to difluoromethylated oxindoles†
Abstract
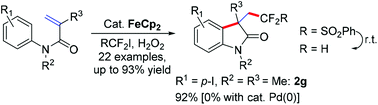
An iron-catalyzed aryldifluoromethylation of activated alkenes under mild reaction conditions has been developed, which is a rare example where a cosolvent is used to improve the reaction yield along with Fenton's reagent and thus provides an economic and green method for the synthesis of a variety of difluoromethylated oxindoles. Preliminary mechanistic investigations indicate a radical addition path.

 Please wait while we load your content...
Please wait while we load your content...