Design, synthesis, and fungicidal activity of novel carboxylic acid amides represented by N-benzhydryl valinamode carbamates†
Abstract
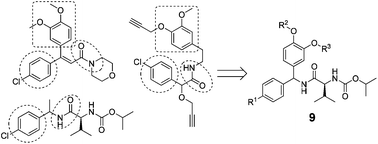
Carboxylic acid amide (CAA) fungicides are an important class of agricultural fungicide with oomycete activity and low toxicity toward mammalian cells. To find CAA analogues with high activity against resistant pathogens, a series of substituted N-benzhydryl valinamide carbamate derivatives were designed and synthesized by introducing substituted aromatic rings into valinamide carbamate leads. Bioassays showed that some title compounds exhibited very good in vitro fungicidal activity against Phytophthora capsici and in vivo fungicidal activities against Pseudoperonospora cubensis. Topomer CoMFA was performed to explore the structure–activity relationship on the basis of the in vitro data. The dimethoxy substituted aromatic analogue 9e was found to display higher in vitro fungicidal activity against Phytophthora capsici than iprovalicarb but lower activity than mandipropamid, and higher in vivo fungicidal activity against Pseudoperonospora cubensis than dimethomorph at a dosage of 6.25 μg mL−1.

 Please wait while we load your content...
Please wait while we load your content...