Asymmetric organocatalytic desymmetrization of 4,4-disubstituted cyclohexadienones at high pressure: a new powerful strategy for the synthesis of highly congested chiral cyclohexenones†
Abstract
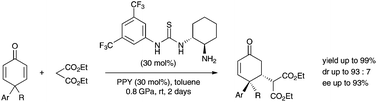
A highly diastereoselective and enantioselective method for the asymmetric desymmetrization of 4,4-disubstituted cyclohexadienones using the Michael addition reaction of malonates under catalysis with the primary amine–thiourea conjugate catalyst and PPY at high pressure was developed.

 Please wait while we load your content...
Please wait while we load your content...