Pd(ii)-catalyzed ligand controlled synthesis of methyl 1-benzyl-1H-indole-3-carboxylates and bis(1-benzyl-1H-indol-3-yl)methanones†
Abstract
A simple change of ligand and solvent allows controlled, effective switching between cyclization–carbonylation and cyclization–carbonylation–cyclization-coupling (CCC-coupling) reactions of 2-alkynylanilines catalyzed by palladium(II). The use of a [Pd(tfa)2(box)] catalyst in iPrOH afforded symmetrical ketones bearing two indoles in good yields; replacing the catalyst and solvent with Pd(tfa)2 and DMSO–MeOH led to the formation of methyl 1-benzyl-1H-indole-3-carboxylates in good yields.
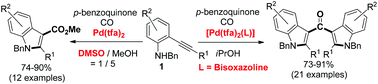
 Please wait while we load your content...
Please wait while we load your content...