Copper(ii)-catalyzed C–O coupling of aryl bromides with aliphatic diols: synthesis of ethers, phenols, and benzo-fused cyclic ethers†
Abstract
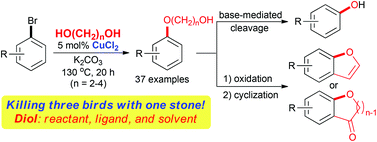
A highly efficient copper-catalyzed C–O cross-coupling reaction between aryl bromides and aliphatic diols has been developed employing a cheaper, more efficient, and easily removable copper(II) catalyst. A broad range of aryl bromides were coupled with aliphatic diols of different lengths using 5 mol% CuCl2 and 3 equivalents of K2CO3 in the absence of any other ligands or solvents to afford the corresponding hydroxyalkyl aryl ethers in good to excellent yields. In this newly developed protocol, aliphatic diols have multilateral functions as coupling reactants, ligands, and solvents. The resulting hydroxyalkyl aryl ethers were further readily converted into the corresponding phenols, presenting a valuable alternative way to phenols from aryl bromides. Furthermore, it was demonstrated that they are useful intermediates for more advanced molecules such as benzofurans and benzo-fused cyclic ethers.
 Please wait while we load your content...
Please wait while we load your content...