N,N-Dimethylaminobenzoates enable highly enantioselective Sharpless dihydroxylations of 1,1-disubstituted alkenes†
Abstract
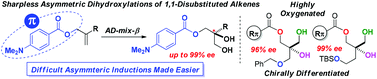
A design scenario aimed at exploring beneficial catalyst–substrate π–π stacking electronic interactions in the classical Sharpless asymmetric dihydroxylations (SAD) leads to the identification of highly polarizable allylic N,N-dimethylaminobenzoate as a remarkably efficient auxiliary for inducing high levels of enantioselectivities (up to 99% ee) in the traditionally challenging substrate class of 1,1-disubstituted aliphatic alkenes.

 Please wait while we load your content...
Please wait while we load your content...