Convergent and enantioselective syntheses of cytosolic phospholipase A2α inhibiting N-(1-indazol-1-ylpropan-2-yl)carbamates†
Abstract
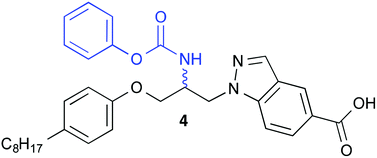
Cytosolic phospholipase A2α (cPLA2α) is an important enzyme of the inflammation cascade. Therefore, inhibitors of cPLA2α are assumed to be promising drug candidates for the treatment of inflammatory disorders. Recently we have found that indole-5-carboxylic acid with a 3-(4-octylphenoxy)-2-(phenoxycarbonylamino)propyl substituent in position 1 is an inhibitor of cPLA2α. We have now synthesized a corresponding derivative with the indole heterocycle replaced by an indazole (4) employing an analogous reaction sequence as for the synthesis of the indole derivative. Besides, a more convergent synthesis for 4 was established using an aziridine as central intermediate. Furthermore, a chiral-pool based enantioselective synthesis was developed for the synthesis of (R)- and (S)-4. Starting compound for both enantiomers was the (R)-serine derived oxazolidine (R)-25. Compound 4 proved to be a moderate inhibitor of cPLA2α, with the S-enantiomer being twice as active as the R-enantiomer. The racemate 4 and the enantiomers (R)- and (S)-4 showed a high in vitro metabolic stability in rat liver S9 fractions.

 Please wait while we load your content...
Please wait while we load your content...