The syntheses of α-ketoamides vianBu4NI-catalyzed multiple sp3C–H bond oxidation of ethylarenes and sequential coupling with dialkylformamides†
Abstract
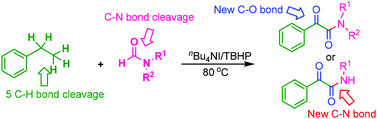
The nBu4NI-catalyzed sequential C–O and C–N bond formation via multiple sp3C–H bond activation of ethylarenes, using N,N-dialkylformamide as the amino source, provided α-ketoamides with moderate yields.
 Please wait while we load your content...
Please wait while we load your content...