One-pot synthesis of 2-amino-4(3H)-quinazolinones via ring-opening of isatoic anhydride and palladium-catalyzed oxidative isocyanide-insertion†
Abstract
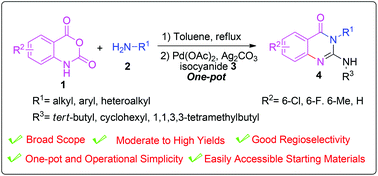
An efficient and practical two-step process has been developed for the synthesis of 2-amino-4(3H)-quinazolinones via ring-opening of isatoic anhydride and palladium-catalyzed oxidative isocyanide-insertion in one pot. This regioselective procedure could construct a wide range of 2-amino-4(3H)-quinazolinones in moderate to excellent yields. Furthermore, the methodology also had distinct advantages of easily accessible starting materials and operational simplicity.

 Please wait while we load your content...
Please wait while we load your content...