A Friedel–Crafts alkylation mechanism using an aminoindanol-derived thiourea catalyst†
Abstract
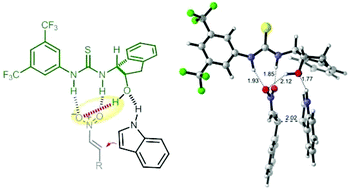
Computational calculations based on experimental results shed light on the mechanistic proposal for a Friedel–Crafts alkylation reaction between indole and nitroalkenes, catalysed by a chiral aminoindanol-derived thiourea. In our hypothesis both substrates are simultaneously coordinated to the catalyst in a bifunctional mode. This study elucidates the crucial role played by the hydroxyl group of the catalyst in the success of the reaction. The OH group seems to be involved in the preferential attack of the indole over the nitroalkene, affording the major enantiomer and stabilizing the resulting transition state by a concomitant coordination with the nitroolefin. The results obtained with other catalysts from the same family, and other indoles, are reported and discussed. Theoretical calculations are in agreement with the experimental outcomes and with our previously developed mechanism, displaying the pivotal role played by hydrogen bond interactions.

 Please wait while we load your content...
Please wait while we load your content...