Organocatalytic asymmetric decarboxylative cyanomethylation of isatins using l-proline derived bifunctional thiourea†
Abstract
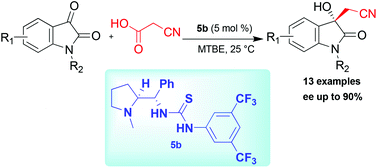
First asymmetric decarboxylative cyanomethylation of isatins is reported herewith using bifunctional thiourea derived from L-proline in good yields and enantioselectivities. This strategy enables the construction of various 3-cyanomethylene substituted 3-hydroxyoxindoles in enantioselective manner. Enantioselective synthesis of CPC-1 alkaloid has been accomplished in fewer steps.

 Please wait while we load your content...
Please wait while we load your content...