Synthesis of homochiral tris-indanyl molecular rods†
Abstract
Homochiral tris-indanyl molecular rods designed for supramolecular surface self-assembly were synthesized. The chiral indanol moiety was constructed via a Ti-mediated alkyne trimerization. Further manipulations resulted in a homochiral indanol monomer. This was employed as the precursor for successive Sonogashira and Ohira–Bestman reactions towards the homochiral tris-indanyl molecular rods. The molecular rods will be applied for scanning tunnelling microscopy studies of their surface self-assembly and chirality.
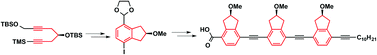

 Please wait while we load your content...
Please wait while we load your content...