Synthesis of functionalized 2-vinyl-2,3-dihydropyrroles and 3-methylene-1,2,3,4-tetrahydropyridines by palladium-catalyzed cyclization of β-enaminocarbonyl compounds with allylic bisacetates†
Abstract
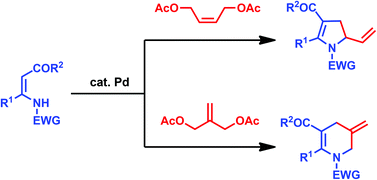
A palladium-catalyzed cyclization of β-enaminocarbonyl compounds with allylic bisacetates is described. 2-Vinyl-2,3-dihydropyrroles and 3-methylene-1,2,3,4-tetrahydropyridines were produced from the reaction of β-enaminocarbonyl compounds with 1,4-diacetoxy-2-butene and 2-methylene-1,3-propanediol diacetate, respectively.

 Please wait while we load your content...
Please wait while we load your content...