Zwitterionic borane adducts of N-heterocyclic carbenes from mesomeric betaines of uracil†
Abstract
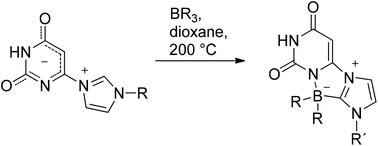
We prepared a series of imidazolium-substituted uracil-anions which are members of the class of cross-conjugated heterocyclic mesomeric betaines. They are in tautomeric equilibrium with their N-heterocyclic carbenes, uracil-6-yl-imidazol-2-ylidenes. These carbenes can be trapped by reaction with sulfur, selenium, as well as by triethylborane and triphenylborane, respectively. The latter trapping reaction yielded the first representatives of a new heterocyclic zwitterionic ring system, imidazo[2′,1′:3,4][1,4,2]diazaborolo[1,5-c]pyrimidinium-10-ide. Results of two single crystal X-ray structure analyses are presented.

 Please wait while we load your content...
Please wait while we load your content...