Direct transformation of arylpropynes to acrylamides via a three-step tandem reaction†
Abstract
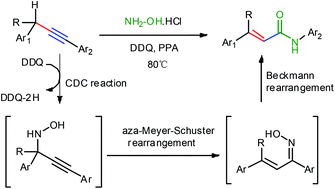
A novel and metal-free acrylamides formation between arylpropynes and hydroxylamine hydrochloride through sp3 C–H and C–C bond cleavage has been achieved with DDQ as an oxidant. The mechanistic study shows that the acrylamides are formed through a three-step tandem sequence, including cross-dehydrogenative-coupling (CDC) reaction, aza-Meyer–Schuster rearrangement and Beckmann rearrangement.

 Please wait while we load your content...
Please wait while we load your content...