Electron transport in DNA initiated by diaminonaphthalene donors alternatively bound by non-covalent and covalent association†
Abstract
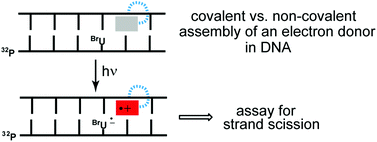
Covalent conjugation is typically used to fix a potential charge donor to a chosen site for studying either hole or excess electron transport in duplex DNA. A model system based on oligonucleotides containing an abasic site and BrdU was previously developed to provide a rapid method of screening new donors without the need of synthetic chemistry. While this strategy is effective for discovering important lead compounds, it is not appropriate for establishing extensive correlations between molecular structure and donor efficiency as demonstrated with a series of closely related electron donors based on diaminonaphthalene. The non-covalent system accurately identified the ability of the donors to reduce a distal BrdU in DNA, but their varying efficiencies were not recapitulated when attached covalently to an equivalent sequence of DNA. Reduction within the covalent system was not sensitive to the strong donor potentials as consistent with charge recombination dominating the net migration of charge.

 Please wait while we load your content...
Please wait while we load your content...