Design, synthesis and properties of artificial nucleic acids from (R)-4-amino-butane-1,3-diol†
Abstract
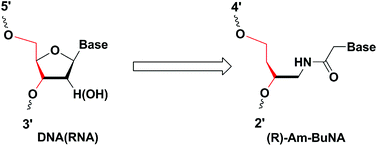
A new artificial nucleic acid analogue, (R)-Am-BuNA, was developed with a simplified acyclic (R)-4-amino-butane-1,3-diol phosphodiester backbone. Phosphoramidite monomers of (R)-Am-BuNA were incorporated into DNA oligonucleotides (ODNs) and G-quadruplexes. Their thermal stability, conformation change and biological stability were further investigated using UV-melting, circular dichroism (CD) and gel electrophoresis. The results suggested that thermal stability of the duplexes of (R)-Am-BuNA modified ODNs and their complementary ODN is highly dependent on the substitution position. Substitution of thymidine at the 7th position in a thrombin-binding DNA aptamer (TBA) results in a slight increase in Tm with no effect on quadruplex conformation on the CD spectrum in comparison to that of the natural G-quadruplex. Further enzymatic experiments with fetal bovine serum (FBS) and snake venom phosphodiesterase (SVPDE) indicated that only single replacement of a (R)-Am-BuNA modified nucleobase greatly inhibited oligonucleotide degradation, which shows their promising applications as capping nucleotides in nucleic acid drugs.

 Please wait while we load your content...
Please wait while we load your content...