Synthesis and in vitro evaluation of tetrahydroisoquinolines with pendent aromatics as sigma-2 (σ2) selective ligands†
Abstract
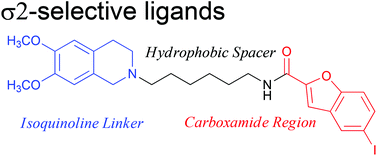
5-Bromo-N-[4-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-butyl)]-2,3-dimethoxybenzamide 1 is a potent and selective σ2 receptor ligand suitable for further development. A series of new analogues, incorporating a variety of isoquinoline and carboxylic acid moieties, linked together with either a linear or cyclic amine spacer have been synthesised and assessed for their σ1/σ2 binding affinity and selectivity. Compounds with a rigid piperidine spacer gave Ki values for the σ2 receptor between 8.7–845 nM. Changing the configuration of the methoxy groups on the isoquinoline moiety resulted in molecules with σ2Ki values of 4.4–133 nM whereas varying the length and flexibility of the carbon spaces gave σ2Ki values 0.88–15.0 nM, some of the most active, selective σ2 ligands to date. Thus, the flexibility and length of the carbon linker and the carboxylic acid moiety are confirmed to be key to the exceptional binding affinity and selectivity for this active series. Additionally, the incorporation of a halogen on selected carboxylic acid moieties provided a convenient strategy for the introduction of a radiohalogen for applications in pharmacological and imaging studies.

 Please wait while we load your content...
Please wait while we load your content...