Synthesis, photophysical and cytotoxicity evaluations of DNA targeting agents based on 3-amino-1,8-naphthalimide derived Tröger's bases†
Abstract
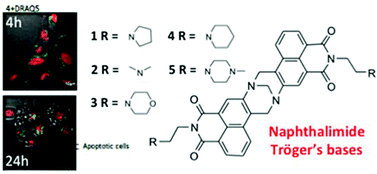
The synthesis and characterisation of five bis-1,8-naphthalimide containing Tröger's bases 1–5 formed from their corresponding 3-amino-1,8-naphthalimide precursors 6–10 is described. The photophysical investigations of 1–5 and 6–10 were carried out in several organic solvents as well as in water and as a function of pH using UV-Vis absorption and fluorescence spectroscopies. The DNA binding affinities of 1–5 in aqueous solution at pH 7.4 were also investigated using several UV-Vis absorption and fluorescence experiments by using calf thymus DNA (ct-DNA). These molecules exhibited significant DNA binding affinities; where large binding values (Kb) in the range of 106 M−1 were determined, even in competitive media (50 mM and 160 mM NaCl at pH 7.4). Thermal denaturation measurements also showed that 1–5 significantly stabilised the DNA helix. Using linear and circular dichroism we further demonstrated that the DNA binding interaction occurs both by intercalation and by groove binding. The Tröger's bases were further shown to be rapidly taken up into cells using confocal fluorescence spectroscopy; and cytotoxic studies in HeLa and MCF-7 cells showed that most of the Tröger's bases were effective cytotoxic agents with EC50 values of between 1.1–12 μM and that all the active compounds induced programmed cell death by apoptosis, where up to 70% cellular death was observed after 24 h of incubation for 4.

 Please wait while we load your content...
Please wait while we load your content...