New facile enantio- and diastereo-selective syntheses of (−)-triptonide and (−)-triptolide†
Abstract
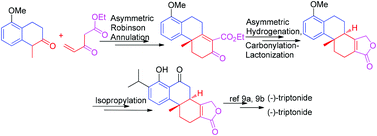
A novel formal asymmetric synthesis of (−)-triptonide and (−)-triptolide, featuring a new alternative access to their known key intermediate 4, has been achieved through two synthetic routes in 9 steps with 13.6% total yield and 10 steps with 18.5% overall yield, respectively. This synthesis is scalable and hence has high potential for application to further synthetic elaboration and biologic investigation on such natural products.

 Please wait while we load your content...
Please wait while we load your content...