Enantioselective bacterial hydrolysis of amido esters and diamides derived from (±)-trans-cyclopropane-1,2-dicarboxylic acid†
Abstract
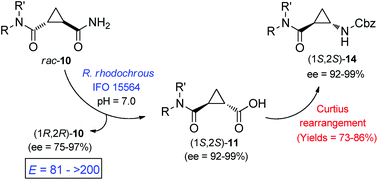
Different optically active amido esters, mixed acid esters, amido acids, and diamides derived from trans-cyclopropane-1,2-dicarboxylic acid were prepared from the commercially available diethyl (±)-trans-cyclopropane-1,2-dicarboxylate. The key step was the Rhodococcus rhodochrous IFO 15564 catalyzed hydrolysis of the corresponding racemic amide. The amidase present in this microorganism showed moderate to high enantioselectivity towards these substrates. In addition a simple and efficient Curtius rearrangement of some of the enzymatically prepared cyclopropanecarboxylic acids allowed us to obtain optically active β-aminocyclopropanecarboxylic acid derivatives with high yields and enantiomeric excesses.

 Please wait while we load your content...
Please wait while we load your content...